|
One of the major obstacles in effectively treating pancreatic ductal adenocarcinoma (PDAC) is its broad, primary resistance to almost all therapies. Current therapies for pancreatic ductal adenocarcinoma (PDAC) are largely ineffective for at least two reasons: 1) poor drug delivery due to ineffective tissue perfusion and 2) a highly immunosuppressive tumor microenvironment. Thus, only a small fraction of chemotherapy in circulation actually accumulates in the tumor. One way to circumvent this issue is to utilize active transport systems to deliver drugs into tumors, instead passive diffusion.
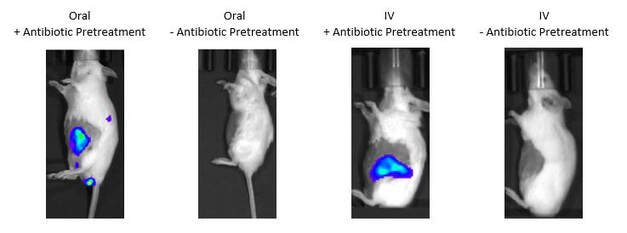
Some types of bacteria can selectively colonize tumors. Bacteria such as E. coli thrive in environments with low oxygen levels, high acidity, and high lactate- exactly the conditions present in pancreatic tumors. In addition, pancreatic tumors have an immunosuppressive microenvironment, so bacteria that would normally be cleared from healthy tissue are able to survive and grow. We have demonstrated colonization of tumors in genetically engineered mouse models that spontaneously develop pancreatic tumors following oral ingestion. |
The advantage of using bacteria for PDAC drug delivery is that while only a small amount may reach the tumor, they multiply to high levels and can be engineered as intratumor bioreactors for the local production of cytotoxic molecules. One promising application is utilizing bacteria to produce immunotherapeutics, which has the potential to overcome some of the major limitations of immunotherapies, such as body-wide immune-related reactions following systemic exposure to the immunotherapy compound.
However, while there has been progress in developing vaccines and checkpoint blockade therapies for PDAC, immunotherapies have only demonstrated consistent responses in a particular subset of PDAC tumors called microsatellite instability-high (MSI-high), which unfortunately account for <1% of all PDAC tumors. In PDAC tumors, not only are T-cells inactivated through checkpoint blockades, they are also kept spatially separated from cancer epithelia by a system of repulsive chemokines. Chemokines, chemical signals that attract and repel different cell types, play an important role in the recruitment of immune cells and directing their migration throughout tissues. CXCL1 and CXCL12 are chemokine signals secreted by cancer associated fibroblasts (CAFs) in PDAC stroma and have been implicated in contributing to this spatial segregation. Although less work has been done to address the tumor-induced local immune suppression as a method of immunotherapy, disrupting these repulsive cytokine signaling pathways may be an effective strategy for relieving the local immune suppression of PDAC tumors and improve the performance of other immunotherapies in combination treatment.
Engineered bacteria can locally produce checkpoint blocking nanobodies and cytokines, and simultaneously act as an adjuvant to prime T-cell activity and boost therapeutic responses. I intend to utilize bacteria to produce and deliver proteins as therapeutics. In addition to cytotoxic molecules that kill tumor cells directly, I also am exploring producing small proteins called nanobodies, a derivative form of an antibody. These nanobodies should inhibit the chemokine receptor CXCR7, potentially negating the effects of the CXCL1 and CXCL12 repulsive chemokine signals and remove immunosuppressive state within pancreatic tumors.
However, while there has been progress in developing vaccines and checkpoint blockade therapies for PDAC, immunotherapies have only demonstrated consistent responses in a particular subset of PDAC tumors called microsatellite instability-high (MSI-high), which unfortunately account for <1% of all PDAC tumors. In PDAC tumors, not only are T-cells inactivated through checkpoint blockades, they are also kept spatially separated from cancer epithelia by a system of repulsive chemokines. Chemokines, chemical signals that attract and repel different cell types, play an important role in the recruitment of immune cells and directing their migration throughout tissues. CXCL1 and CXCL12 are chemokine signals secreted by cancer associated fibroblasts (CAFs) in PDAC stroma and have been implicated in contributing to this spatial segregation. Although less work has been done to address the tumor-induced local immune suppression as a method of immunotherapy, disrupting these repulsive cytokine signaling pathways may be an effective strategy for relieving the local immune suppression of PDAC tumors and improve the performance of other immunotherapies in combination treatment.
Engineered bacteria can locally produce checkpoint blocking nanobodies and cytokines, and simultaneously act as an adjuvant to prime T-cell activity and boost therapeutic responses. I intend to utilize bacteria to produce and deliver proteins as therapeutics. In addition to cytotoxic molecules that kill tumor cells directly, I also am exploring producing small proteins called nanobodies, a derivative form of an antibody. These nanobodies should inhibit the chemokine receptor CXCR7, potentially negating the effects of the CXCL1 and CXCL12 repulsive chemokine signals and remove immunosuppressive state within pancreatic tumors.