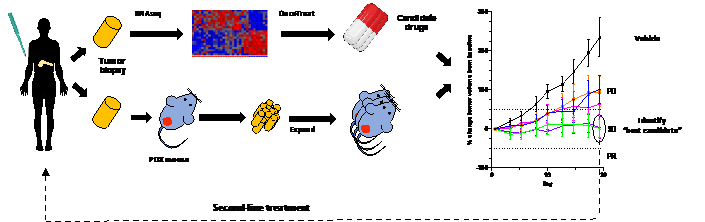
Pancreatic ductal adenocarcinoma (PDA) is the third most common cause of cancer mortality, with a five-year survival rate of just 8%. Although PDAC is genetically characterized by high penetrance alterations in four genes (K-ras, p53, cdkn2a, and smad4), none of them can be targeted therapeutically. Indeed, only a small fraction of pancreatic tumors harbor any “targetable” genetic alterations, suggesting that the large majority of patients will not benefit from precision medicine with the current DNA-based paradigm of oncogene dependence. Recently, the Califano laboratory at Columbia University Irving Medical Center have developed a novel RNA-based precision medicine framework called OncoTreat, which is being translated in collaboration with the Olive lab. Based on an area of systems biology called regulatory network analysis, OncoTreat enables the identification of Master Regulator (MR) proteins that drive the malignancy of individual tumors. Patients are then matched to drugs by screening for agents that impact regulatory activity by opposing the specific MRs active in the patient’s tumor. This concept will be evaluated in a Phase 1b clinical trial at Columbia Presbyterian Hospital. I propose to perform a co-clinical evaluation of this novel form of precision medicine, using personalized models from each subject, to identify key MRs for each tumor, predict matching drugs, elucidate the mechanism of action and then treat the models with top candidate agents. The most successful treatments will be provided back to the patient in second line (Figure1). Finally, samples and data from this study will be used to address biological questions about determinants of sensitivity to treatment, mechanisms of response to therapy, and the impact of standard chemotherapy on MR profiles. Thus, this proposal will bring a comprehensive alternative strategy for precision medicine of PDAC patients.
Figure1. PDA tumor biopsies will be RNA sequenced and analyzed by OncoTreat to predict the best candidate drugs for the top MRs. Additional biopsy material will be implanted in NSG mice to generate patient derived xenografts for co-clinical evaluation of top regimens predicted by OncoTreat. Results of these experiments will be returned to the clinic to aid in selection of agents for patients in the second line setting.